Oxidative bleaching under high temperature, high PH value or longer time conditions is mostwidely used to achieve the desired efifect, but these conditions can cause fiber damage. Compared with the traditional hydrogen peroxide bleaching, bleaching cotton fabric with H2O2 activator as crystal is a vital innovation. It can reduce the temperature of hot bleaching or shorten the padding time of pad-batch cold scouring. Meanwhile it can reduce the temperature or shorten bleaching of cold rolling mill of practice bleaching piled up time, and improve the quality of products. In this paper, TBCC , a new hydrogen peroxide activator, was prepared and the low temperature oxidation system was constructed for cotton and its blend fabric bleaching according with the requirements of both environmental protection and stability. The low temperature bleaching process was developed and the available attempt for energy saving and bleaching problem was explored.
The synthesis of N-4-trethylammonometh ) benzoyl caprolactam chloride ( TBCC ) , an activator of hydrogen peroxide bleaching was studied . The synthetic routes were discussed . At last ,the products structure was verified by FTIR analysis . On the basis of TBCCS preparation , the application of H O / BCC low temperature oxidation system in bleaching of cotton fabric was investigated . The activating effect of TBCC on H O bleaching was investigated and themechanism was analyzed . Furthermore , in order to reduce the application cost , a few kind of TBCC compound systems were also investigated.
The synthesis of TBCC was divided into two steps. First, 4-(chlorometh l) enzo caprolactamwas synthesized by the reaction of 4-(chlorometh )benzyl chloride and caprolactam with methylbenzene as dissolvent. The optimal conditions were: 4-(Chloromethyl)benzoyl chloridewas slowly added to hot blends of caprolactam and methylbenzene, the mole ratio being 1: 1.5dosage of triethylamine was the same as4-hloromethy)benzoyl chloride, temperature 110℃,time 6h . Second , TBCC was finally synthesized by the reaction of 4- ( Chloromethyl ) benzoylcaprolactam and triethylamine with acetonitrile as dissolvent . The suitable synthesis condition were : the mole ratio of 4- ( Chloromethyl ) benzoyl caprolactam and triethylamine was 1 : 15temperature 90℃ , time 4h . The chemical structure of TBCC is as following
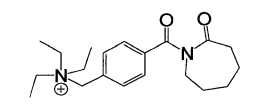
TBCC as an activator could significantly improve the whiteness of fabric at a lower temperature and without any stabilizing agent . The optimal conditions in this activated H2O2 bleaching system was confirmed by orthogonal experiments , temperature 70℃ , H202 3g / L , NAOH Ig / LTBCC 4g / L , time 55min
TAED could effectively activate the H2O2 bleaching system at a lower temperature and PH value . Mixed TAED and TBCC got better whiteness , because the two active agents had a synergistic effect . Urea , caprolactam with TBCC and the grafting agent with TBCC were applied.
When the fabric modified by the cation bleached at low-temperature system , the whiteness of fabric was improved . The dye uptakes and K / S of modified fabric increased rapidly in the early stages of infection , much higher than the fabrics without any treatment The dyeing properties ofthe fabric modified by the cation were not changed after bleached by H2O2.

