So far, the most common cellulose fiber bleaching systems used in the dyeing and finishing industry all over the world are no more than the following three: system, sodium chlorite system and sodium hypochlorite system in acidic medium. Although sodium hypochlorite has good bleaching effect and almost no damage to cellulose, it has high requirements for equipment, high bleaching cost and great limitations in the application of bleaching technology, and the sodium hypochlorite bleaching system is facing the fate of elimination because of the serious pollution of adsorbable organic dentate (1). With the strengthening of people’s awareness of environmental protection, especially after the adsorbable organic dentate is listed as the wastewater discharge index, the advantages of hydrogen peroxide bleaching process are further strengthened. Hydrogen peroxide is an excellent bleaching agent. It is welcomed by printing and dyeing enterprises because of its short treatment time, high whiteness, no yellowing, low environmental pollution and wide application range; And with the traditional hypochlorite as the representative of the bleaching agent, continuous progress has been made
Properties of hydrogen peroxide
The chemical formula of hydrogen peroxide is H2O2. Its aqueous solution, also known as hydrogen peroxide, is colorless and tasteless. It is a strong oxidant, but if it encounters an oxidant with stronger oxidation than it, it has the property of reducing agent. Hydrogen peroxide is extremely unstable and will gradually decompose and release oxygen during placement. It decomposes faster after heating, illumination or adding catalyst. The catalyst includes divalent iron ion, copper sulfate, hydrogen iodide, complex enzyme and so on. The decomposition formula is
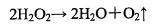
Hydrogen peroxide is a weak dibasic acid, which can be ionized in aqueous solution as shown below
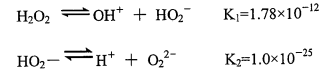
The composition of hydrogen peroxide solution is complex, and the solution composition changes with the change of pH value. When the alkali agent is added into the solution, the H ‘generated by neutralization and ionization will promote the ionization reaction and generate more HO2. The higher the pH value, the more HO2. Until the pH of the solution is > 11, most of the hydrogen peroxide in the solution exists as hydrogen peroxide anion (HO2). Therefore, the stability of the solution is very poor at this time, and the stability decreases with the increase of pH value. When the pH value is low (< 4), the hydrogen peroxide solution is relatively stable. When the pH value is close to 5, the hydrogen peroxide begins to decompose, and the decomposition rate accelerates with the increase of pH value. In a word, hydrogen peroxide is easy to decompose under alkaline conditions, but it is relatively stable under weak acid conditions. Therefore, commercial hydrogen peroxide has added acids as stabilizers.
Hydrogen peroxide bleaching mechanism
The bleaching mechanism of hydrogen peroxide has not been determined so far. At present, the dyeing and finishing industry generally agrees that hydrogen peroxide ion (Hoo) is the active substance of hydrogen peroxide bleaching. In addition, the free radicals released during its decomposition also play a certain role in bleaching. In alkaline hydrogen peroxide bleaching solution, there is a reaction as shown in the formula
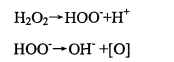
It is generally believed that when the pH is greater than 11.5, most hydrogen peroxide molecules exist as HOO-. According to quantum theory and molecular orbital theory, the color of organic matter is due to the existence of mobile π electron spoon in the conjugated double bond system. The free radical or negative ion produced by the decomposition of hydrogen peroxide attacks or reacts with the colored system, so that the original conjugated system in the pigment is interrupted, the movement range of π electron becomes smaller, the conjugated system becomes shorter, and the color of the object changes from dark to light until fading 2. Due to the instability of HOO-, it may also be decomposed into hydroxyl ions and primary oxygen according to reaction (1-5). This reactive oxygen species can react with the double bond of pigment chromophore to produce achromatic effect and bleaching effect. It can be concluded that HOO- is the main active substance.
Present situation of hydrogen peroxide bleaching
At present, the application scope of hydrogen peroxide bleaching is gradually expanding. Hydrogen peroxide bleaching process should be used in cold pad batch, short flow pretreatment and bleaching of high-grade textiles. In addition, hydrogen peroxide can be used for bleaching cellulose fibers, protein fibers and their blended fabrics with chemical fibers. Compared with sodium hypochlorite and sodium chlorite, the bleaching wastewater does not contain available chlorine and will not pollute the environment; In addition, it has a wide range of applications. It can not only adopt a variety of processing processes, such as dip bleaching, drench bleaching, roll bleaching, etc., but also be treated in the same bath with desizing and refining, combining two or three processes (desizing and bleaching) into one step. The development of new hydrogen peroxide bleaching process has promoted the emergence of corresponding new additives and equipment. However, this does not mean that the hydrogen peroxide bleaching technology has reached a “perfect” level. There are two problems to be solved in practical application.
On the one hand, hydrogen peroxide is unstable. It is relatively stable only under neutral or weakly acidic conditions. It is very easy to decompose under alkaline conditions. If there are alkali metal ions, especially transition metal ions, the decomposition will be accelerated, resulting in the decline of hydrogen peroxide bleaching efficiency and fabric damage. Therefore, in order to promote the effective decomposition of hydrogen peroxide, avoid fiber damage and achieve good bleaching effect, in addition to controlling the pH value of the bleaching solution, substances that effectively remove the transition metal ions in the bleaching solution and control the distribution of metal ions in the bleaching solution must be added before H2O2 bleaching. On the other hand, according to the current hydrogen peroxide bleaching process:
(1) Hydrogen peroxide steaming (or cooking) process: after high temperature treatment for a long time, not only the energy consumption is high, but also the fiber is greatly damaged, which is manifested in the serious decline of fabric strength, especially the decline of protein fiber and new alkali resistant fiber.
(2) Hydrogen peroxide cold pad batch process: the fluctuation of dip rolling amount, stacking time, ambient temperature and washing conditions have a great impact on the bleaching effect, and the quality stability of its products needs to be improved.
For the above shortcomings, seeking suitable technical solutions for improvement has become the focus of hydrogen peroxide bleaching research.

