As we all know, biological enzymes have the advantages of high efficiency, specificity and mild conditions. In recent years, it has been found that using some peroxidases as catalysts in low-temperature bleaching of cotton fabrics has a good effect. The development of enzyme is similar to that of biological simulation, but the stability of enzyme is poor. The structure of the simulated enzyme designed and synthesized is a molecular aggregate or non protein molecule with simple and stable structure than that of the natural enzyme. At present, metal complexes with application potential mainly include metal phthalocyanine complexes, metal porphyrin complexes, nitrogen-containing macrocyclic metal complexes, saltren complexes, Schiff alkali metal complexes, etc.
Metal phthalocyanine complex
Metal phthalocyanine complexes are a class of compounds with macrocyclic conjugated structure, and the structural formula is shown in the figure. These compounds have high stability and good acid, alkali, heat and sun resistance. In addition, phthalocyanine compounds have excellent properties in conductivity, chemical catalysis, photoelectric effect, liquid crystal display, gas sensitive effect, phototherapeutic drugs, photochromic and even nonlinear optical materials. At the same time, metal phthalocyanines and their derivatives also have the catalytic activity and selectivity similar to enzymes, so they are suitable as catalysts for redox reactions.
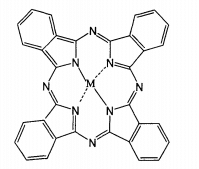
Metalloporphyrin complex
Metalloporphyrin complexes are the general name of coordination compounds formed by porphyrins and their derivatives (porphyrins) and metal ions. Porphyrin is a macroplanar molecule composed of four pyrrole rings linked by conjugated double bonds, in which four nitrogen atoms contain lone electron pairs, which can be combined with metal ions to synthesize metalloporphyrins of macrocyclic conjugated system. The structural formula is shown in the figure.
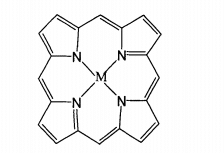
Nitrogen containing macrocyclic metal complexes
Macrocyclic compounds are cyclic compounds formed by polydentate ligands containing oxygen, nitrogen, sulfur, phosphorus and other coordination heteroatoms on the skeleton of the ring. Among them, nitrogen-containing macrocyclic complexes with unique spectrum, structure, photoelectromagnetic chemical properties, kinetic and thermodynamic stability have attracted extensive attention, and there are more and more studies on these complexes. At the same time, nitrogen-containing macrocycles can be complexed with most metal ions, and the coordination speed is fast.
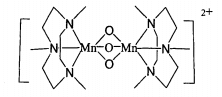
Macrocyclic polyamine is a kind of nitrogen-containing compounds. It contains multiple nitrogen atoms on its cyclic skeleton composed of carbon atoms. Its multi nitrogen coordination environment and macrocyclic effect can be used to construct the center of metal mimic enzyme. In particular, the coordination of macrocyclic triamine compounds with metal ions adopts a surface structure. When used as a metal enzyme simulant, it is easy to form a catalytic active vacancy, so the resulting metal complexes are usually very stable 1411. It is reported in the literature that the binuclear manganese complex of macrocyclic triamine 1,4,7. Trimethyltriaazacyclononane (mnme3tacn for short), with the structural formula shown in the figure, is an efficient catalyst. The complex has excellent catalytic performance for hydrogen peroxide at low temperature and can effectively remove the dirt on the fabric. It has been added to washing powder and developed into a commodity.
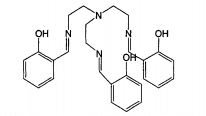
Saltren complex
The structure of saltren complex is shown in the figure.
It is found that the complexes formed by metal manganese and a series of saltren ligands have strong activity, especially the complexes of unsubstituted saltren ligands and trivalent manganese have the strongest activity. Saltren. Mn Complex can effectively catalyze bleaching at a lower temperature of 20 ℃ ~ 30 ℃. The treated fabric has good safety, prevents white graying, and can coexist with enzymes. Yang Yadish et al studied the application of N, n ‘, N. tris (salicylaminoethyl) ammonia manganese complex (saltren. Mn) to the bleaching process of cotton knitted fabrics, which can effectively improve the whiteness of cotton fabrics and reduce the bleaching temperature.
Schiff alkali metal complex
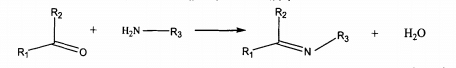
Schiff base is a kind of organic compound containing imino (HC -. N) or alkylimino (RC = n). This kind of compound was first discovered by schiftf in 1864. It is mainly formed by condensation of two kinds of substances containing active carbonyl and amino groups. The general reaction formula is shown in the formula.
It is found that the manganese complex of Schiff base has a good use in textile low-temperature bleaching. It can effectively remove stains and spots on the fabric at room temperature, has a certain disinfection and sterilization effect, has little damage to the dyes and fibers on the fabric, and can effectively prevent the re deposition of migrating dyes. Yuan Shujun studied the application of Schiff base copper complex of salicylaldehyde bovine xanthate in hydrogen peroxide bleaching of cotton fabric. The results show that the bleaching temperature can be reduced, the whiteness of the bleached fabric is better and the strength damage is less.

