In the traditional scouring and bleaching process of fabric, caustic soda acts together with hydrogen peroxide and surfactant to produce a series of chemical reactions with impurities on the fabric, making them soluble products, which are removed under the follow-up hot water and mechanical action. In particular, the catalytic effect of caustic soda on hydrogen peroxide is particularly important. Therefore, it is necessary to explore the influence of caustic soda dosage on the scouring and bleaching effect of cotton fabric. Under the following conditions: dm-1377 2G / L, dm-1430 3G / L and dm-1130 1g / L, dm-1436 2.5g/l and dm-1130 1g / L, dm-1435 0.25g/l and dm-1130 1g / L, the common conditions of the four types of processes are 35% H2O2 12.5g/l, bath ratio 1:10, 60 ℃ 60min, and the pH value range of the treatment solution is controlled to 7 ~ 12, The drawing is as follows
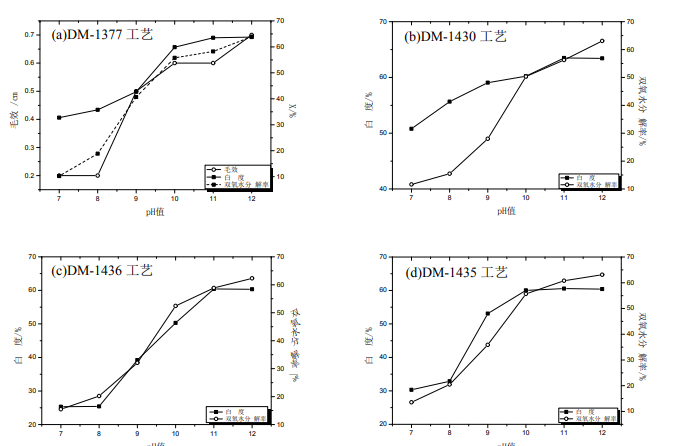
It can be seen from the figure that the pH value of the treatment solution has a significant impact on the low-temperature scouring and bleaching process. In the four kinds of low-temperature scouring and bleaching processes, with the increase of caustic soda concentration in the treatment solution, the whiteness of fabric and the decomposition rate of hydrogen peroxide in the treatment solution are increasing in each process. Before pH 10, the whiteness increases greatly, and the decomposition rate of hydrogen peroxide also increases greatly. The reason may be that with the increase of the pH value of the solution, the Hoo – concentration of the system increases, and the low-temperature scouring and Bleaching Auxiliaries react with Hoo – quickly to form bleaching substances, so that the whiteness of the fabric increases rapidly, and the whiteness growth rate slows down when the pH value is between 10-12. Therefore, the appropriate pH of the treatment solution of each low-temperature scouring and bleaching process can be controlled to be 10 ~ 12.
When the pH value is 10-12, it can be seen from the figure that the whiteness of dm-1436 and dm-1435 decreases slightly,The reason may be that they belong to hydrogen peroxide catalysts with chelated metal ions. With the increase of pH, some metal ions will form hydroxides due to the strong alkalinity of the system. However, due to the small amount, no precipitation is formed. They exist as hydrate colloidal substances of some metal hydroxides, blocking Hoo – inhibits its bleaching.
In addition, in terms of wool effect, except for dm-1377 process, the other three low-temperature scouring and bleaching processes basically have no wool effect. It is possible that dm-1377 contains scouring agent to improve wool effect. Even dm-1377 process has poor wool effect, and the highest wool effect is 0.7cm at pH 12. It may be because the melting point of cotton wax is 68 ~ 80 ℃, and the temperature conditions of the system are not enough to remove a large number of hydrophobic substances such as pectin and cotton wax that affect wool effect, Thus, the wool effect of each low-temperature scouring and bleaching process is very poor.
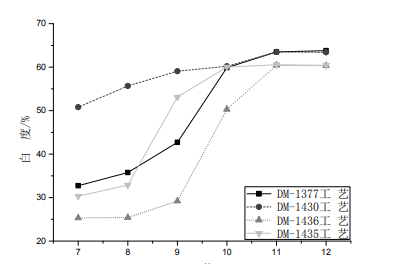
In order to further understand the influence of the pH of the treatment solution of each low-temperature scouring and bleaching process on the whiteness of the fabric, it can be seen from the figure that with the increase of the pH of the treatment solution, the change trend of the whiteness of the fabric treated by each method is basically the same, the pH value is between 10-12, and the whiteness growth rate slows down. Therefore, the appropriate pH of the treatment solution of each low-temperature scouring and bleaching process can be controlled to be 10-12.

