The traditional alkali oxygen bleaching system of cotton fabric has some disadvantages, such as high energy consumption, strong damage and so on. The oxidation potential of peracetic acid in peroxy acid bleaching system is higher than that of, and the activation energy is lower than that of hydrogen peroxide. It can be activated at lower temperature, so as to reduce the bleaching temperature. However, peracetic acid is easy to explode when heated, which limits its industrial production. Inspired by this, the compound generated by grafting acyl chloride or anhydride onto oxygen-containing, sulfur-containing or nitrogen-containing atoms is hydrogen peroxide activator, which can react with hydrogen peroxide to form acyl compounds and decompose dissociation radicals.
Aminonitrile amphoteric activator
Betaine amide acetaminonitrile chloride (tsja) belongs to this kind of activator. It is formed by the reaction of betaine with aminoacetonitrile after chlorination by dichlorosulfoxide. It also belongs to quaternary ammonium salt cationic compounds. Due to the introduction of cyanamide, hydrogen peroxide anion can attack the unstable cyano group and produce imine peroxide anion with strong activity. The reaction mechanism is shown in the formula.

According to the formula, imine peroxide anion has two attack points: cyano carbon atom and acyl carbon atom. It is difficult to attack acyl groups due to the departure of macromolecules and the existence of space obstacles; It is easy to attack the cyano group without leaving the radical, and can form imino peroxy acid anion. Therefore, the compound has greater bleaching activation activity and better low-temperature bleaching effect.
Sugar bleaching activator
Acylates of sugar and its derivatives can also be used as a kind of bleaching activator. Sugars come from a wide range of sources and are renewable. They can be easily transformed into bleaching activators by acylation reaction. Such activators can be prepared from monosaccharides, polysaccharides, oligosaccharides and glucose in monosaccharides. For example, pentaacetylglucose (PAG) is obtained by reacting acetic anhydride with glucose and then removing part of acetic acid. The structural formula of pentaacetylglucose is shown in the figure.
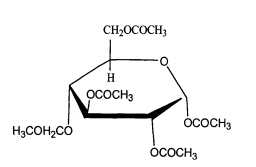
PAG is a promising surfactant. Its bleaching activation mechanism is almost the same as that of the above substances. Its own acetyl group and hydrogen peroxide can produce more oxidizing peracetic acid, which has a high bleaching efficiency activity. Although PAG has broad application prospects, its synthesis and application cost are relatively high, and there is less research in this direction in China. Similarly, by oxidizing and decomposing the diol in the polysaccharide structure to obtain the carboxylic acid group and then acylation, the bleaching activator of polysaccharide can be obtained. This kind of activator has high yield, simple post-treatment and easy purification.

